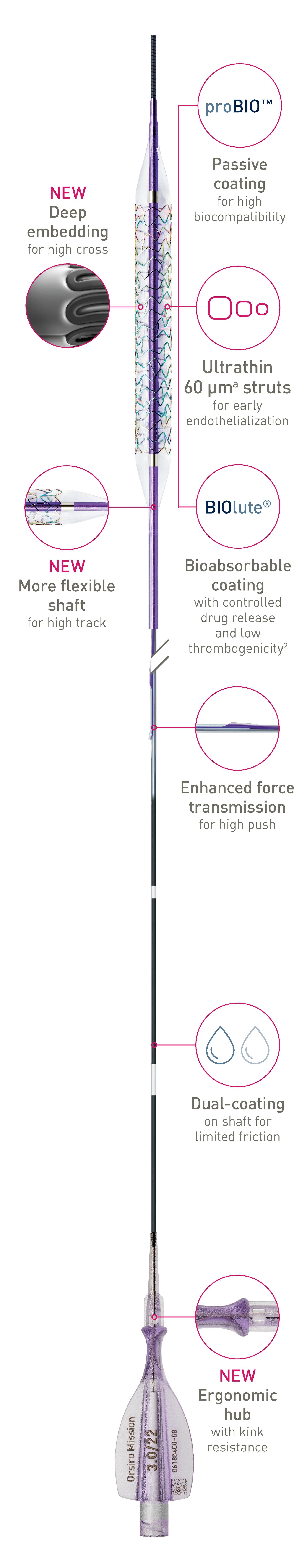
Full system
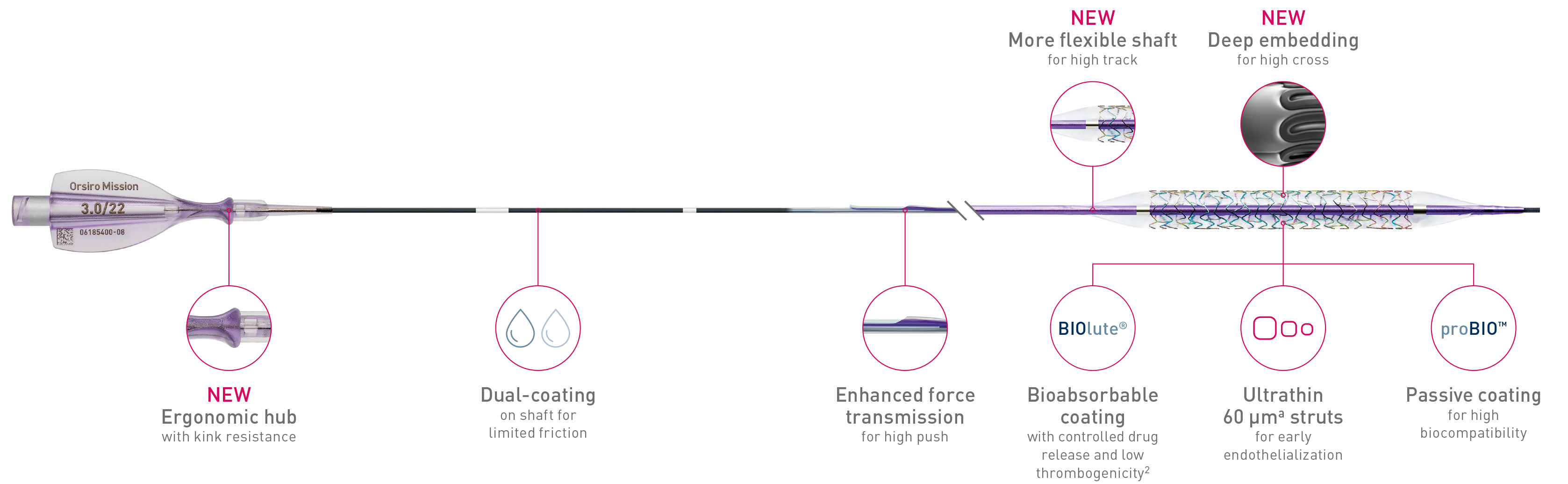
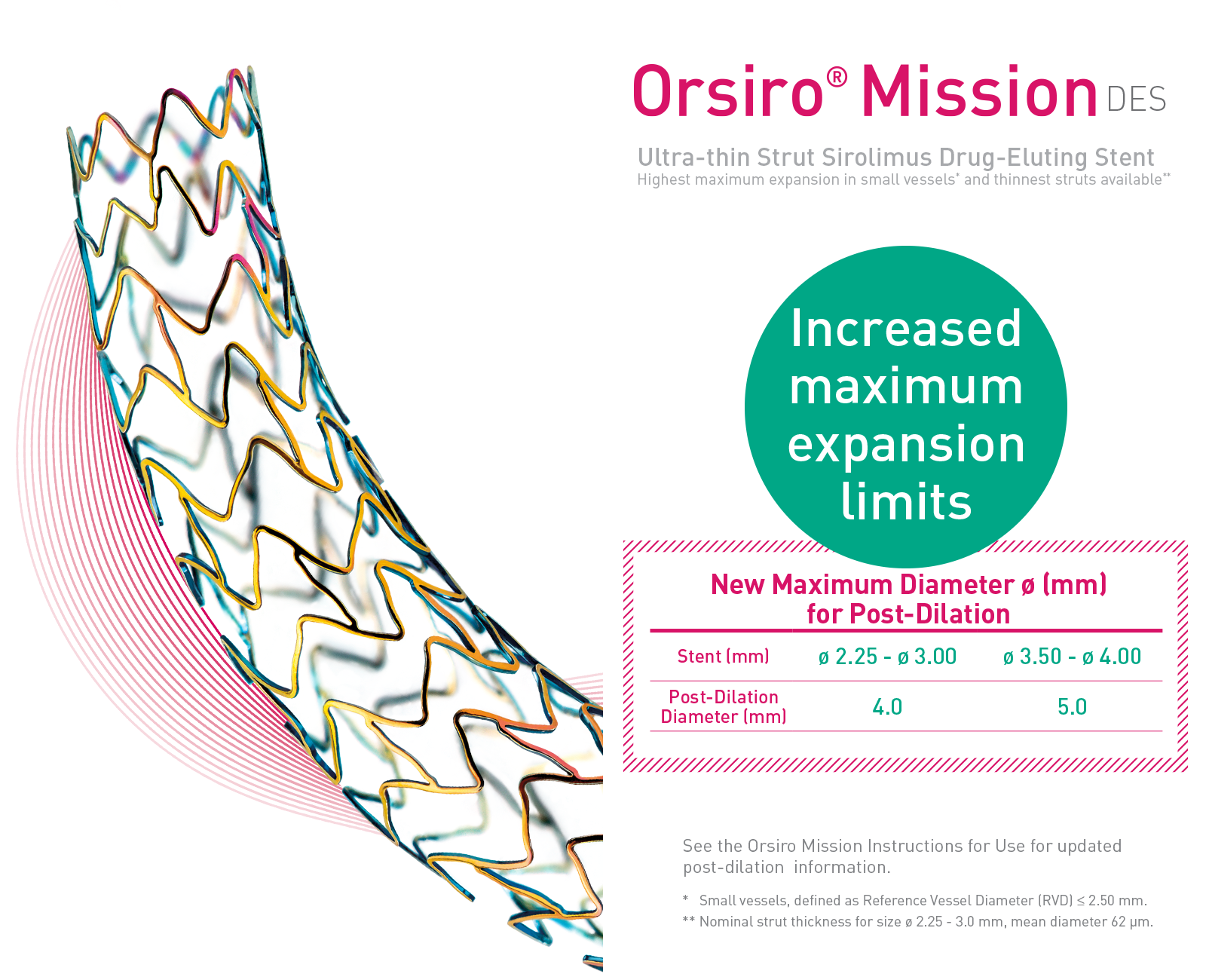
The next level of deliverability1
1st in Push3
Transmits more force from hub to tip.


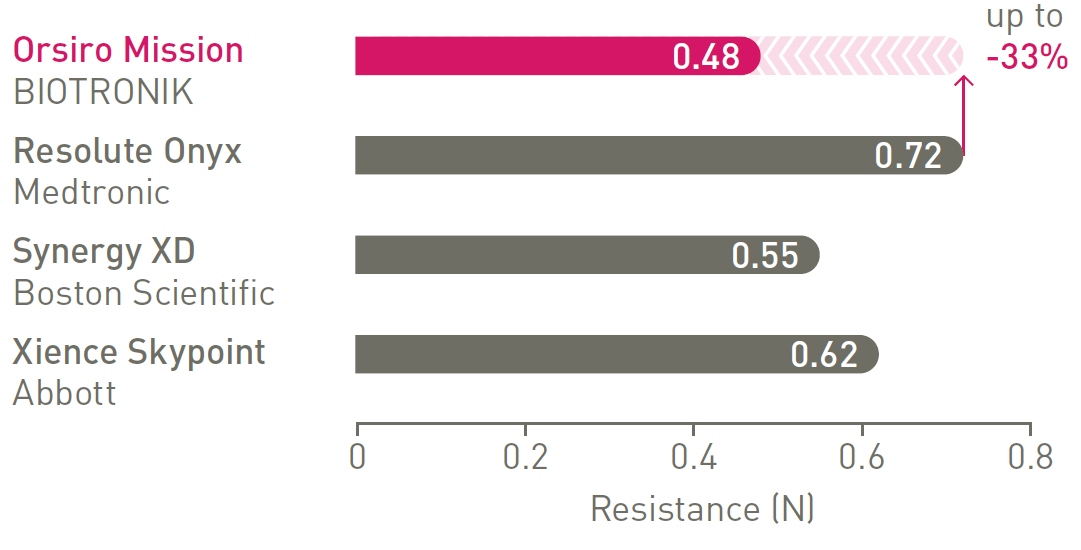
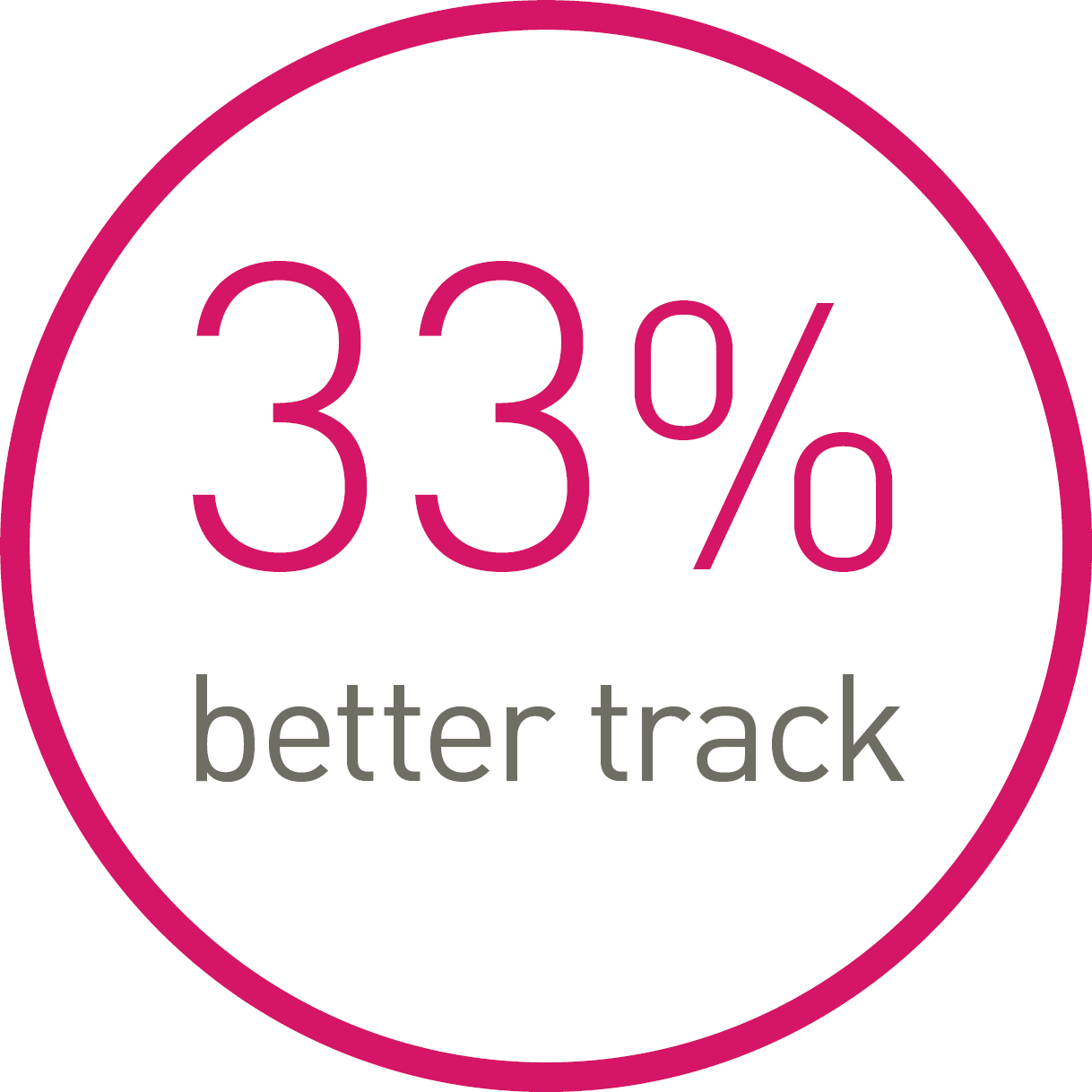
1st in track3
Less force needed to follow the path.
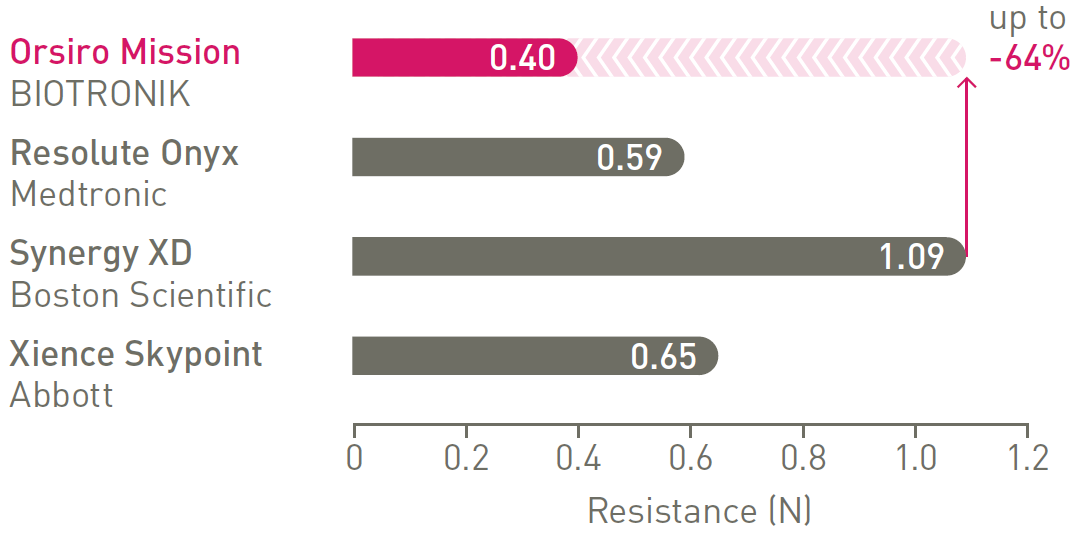
1st in Cross3
Less force needed to successfully cross demanding anatomies.

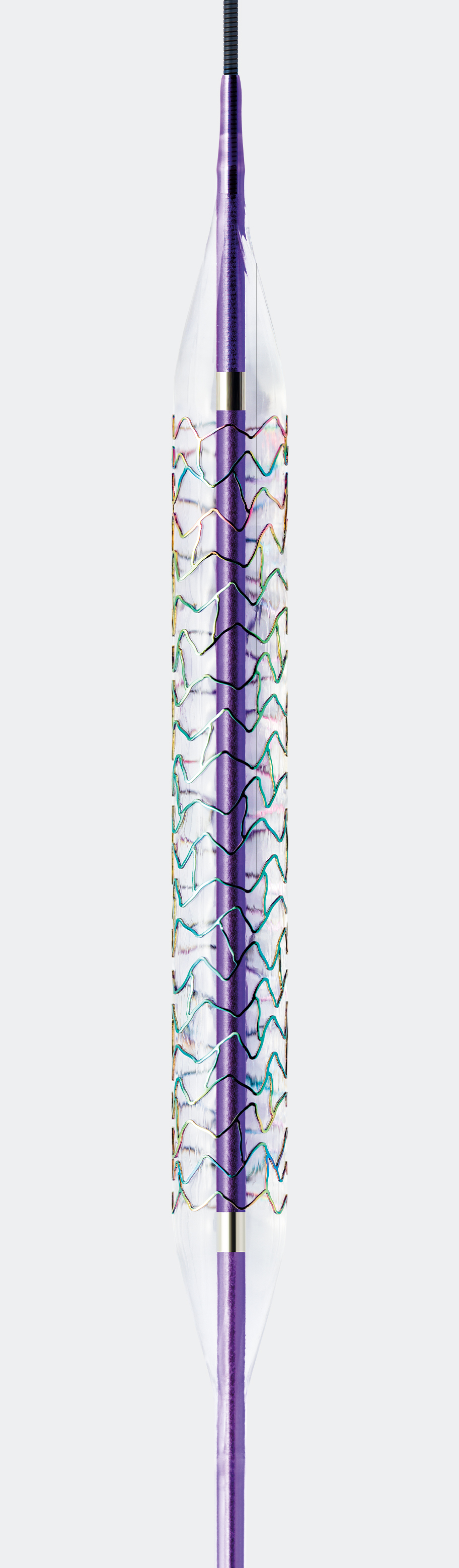

Ultrathin struts4,a – thinnest available in the US5
For early endothelialization
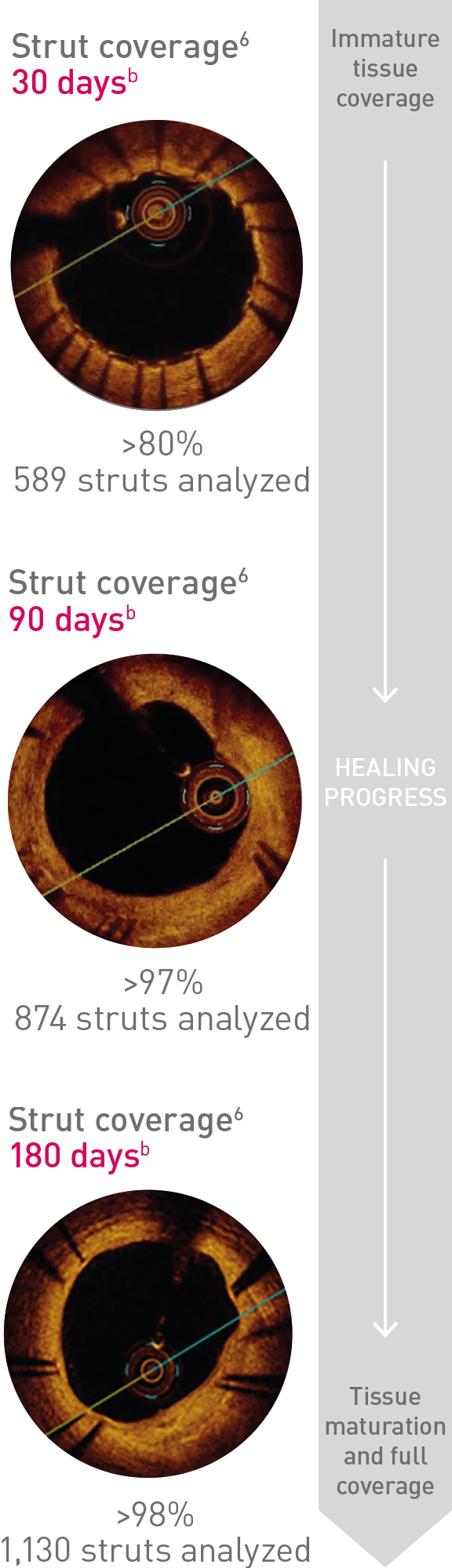
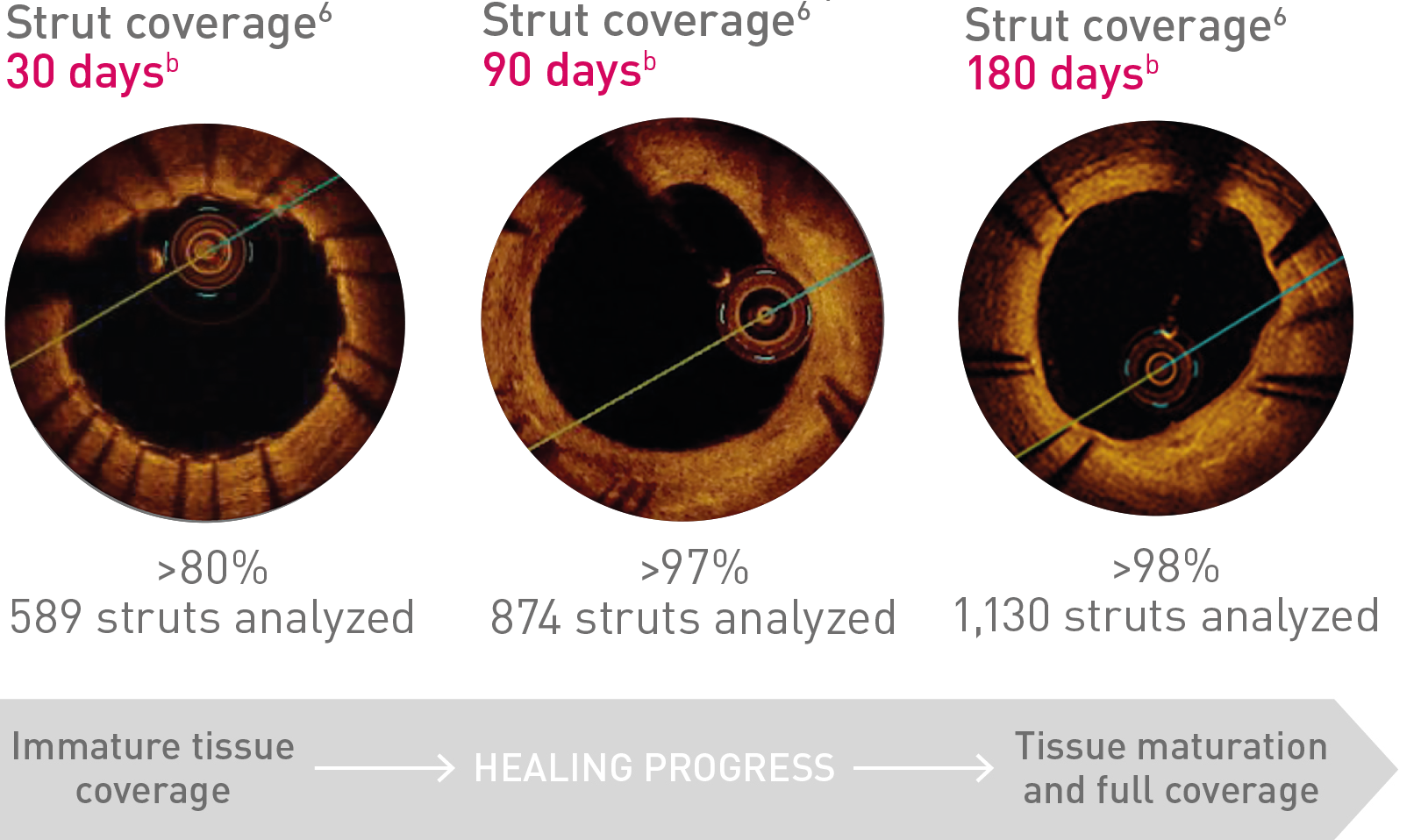
BIO-RESORT Small Vessels
(n = 1,506)
(n = 1,506)
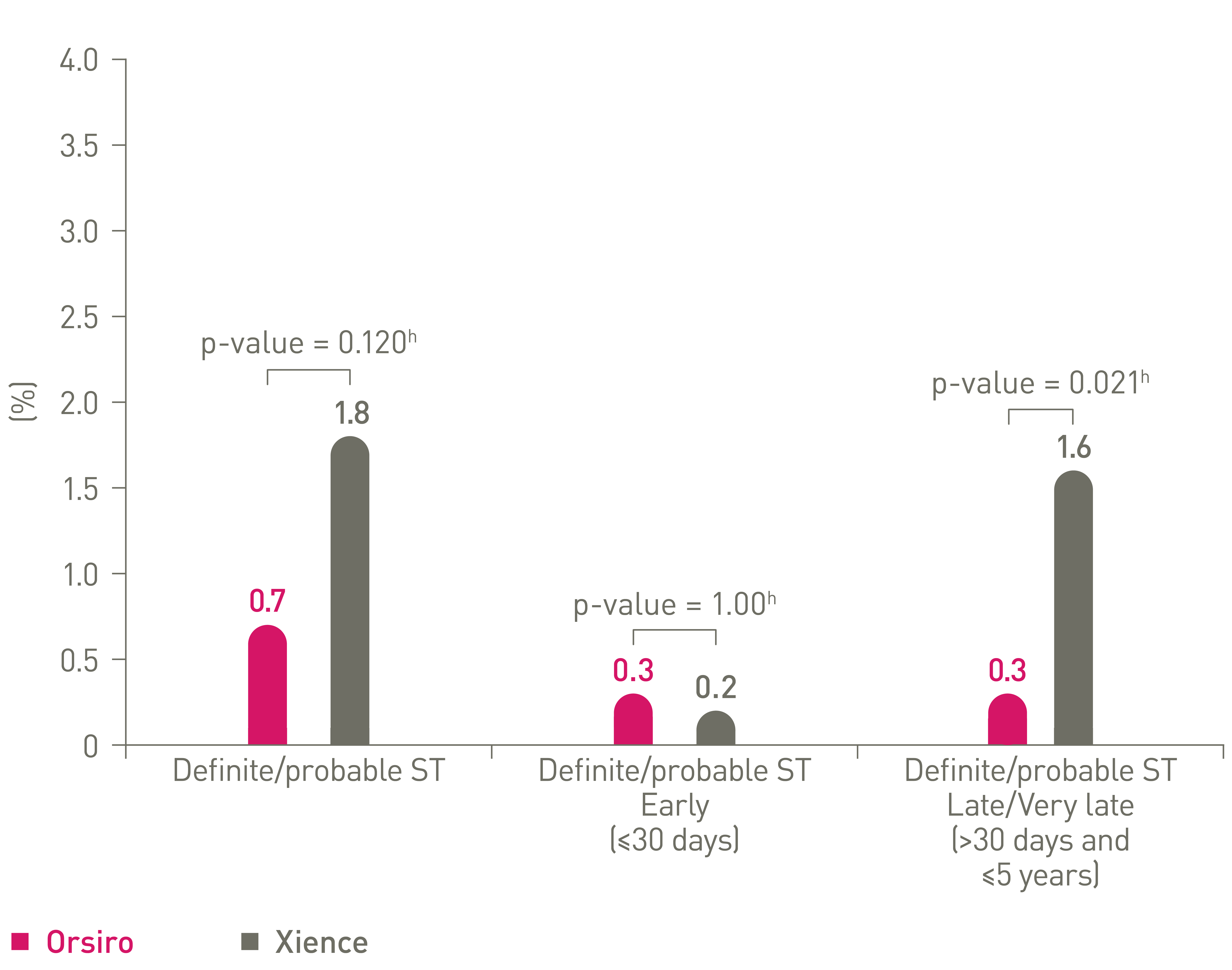
Low stent thrombosis (ST) at 5 years11
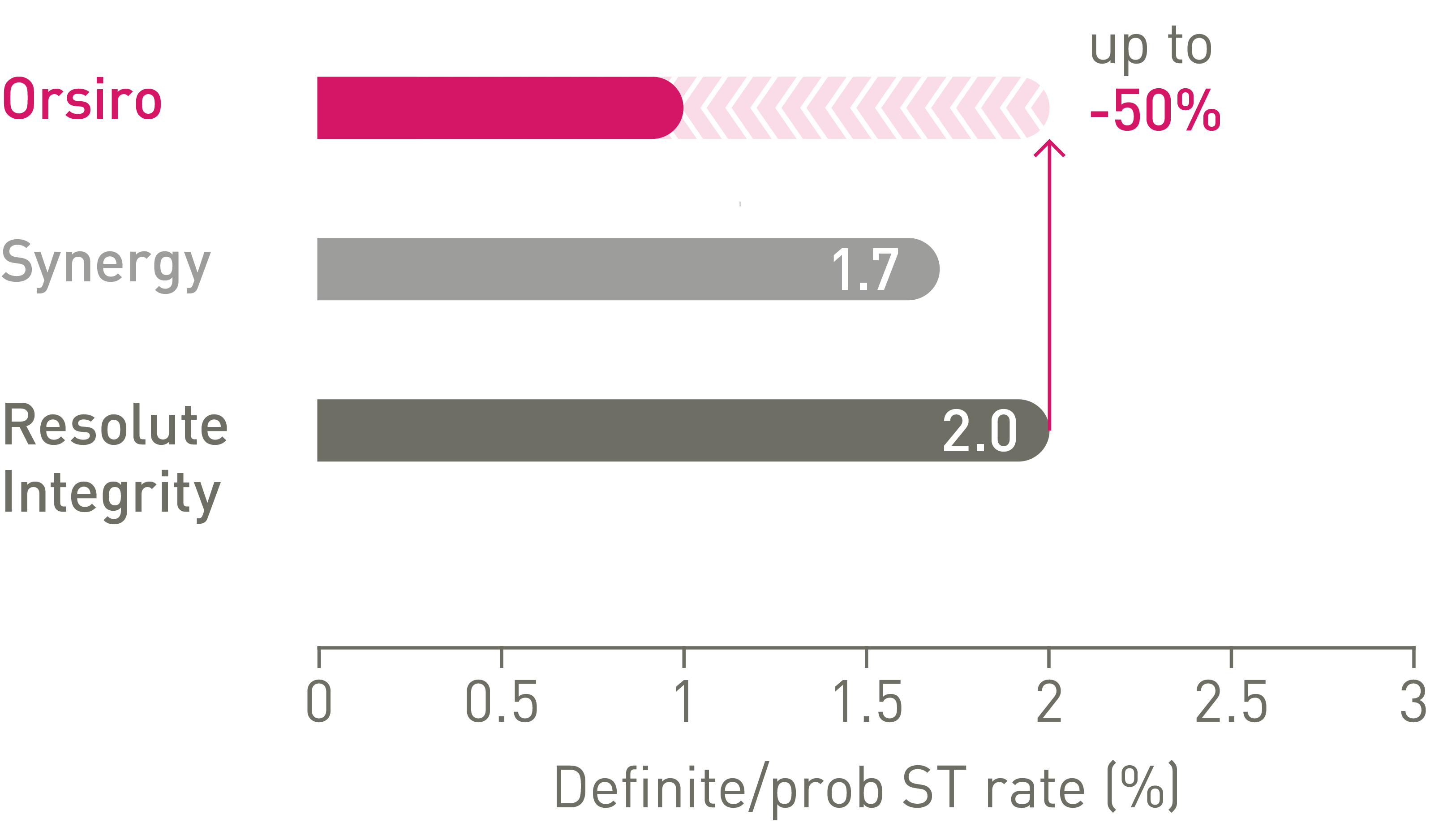

Thinner struts make the difference7
• Less disrupted flow
• Improved re-endothelialization
• Less disrupted flow
• Improved re-endothelialization
Strut thickness in perspective8

BIOTRONIK
CoCr-SES 60 μma

Boston Scientific
PtCr-EES, 74 μm

Medtronic
CoNi-ZES, 81 μm

Abbott
CoCr-EES, 81 μm
Outstanding patient outcomes12
ONE OF THE MOST STUDIED DESd

| STUDY NAME | STUDY TYPE | PATIENTS | STATUS | PRIMARY ENDPOINT |
|---|---|---|---|---|
| BIOSTEMI | RCT | 1,300 | 24-Month FU available | TLF at 12 Months |
| TAGLIERI et al. | Network Meta-Analysis | 99,039 | - | TLF at 12 months and the longest FU available |
| BIOFLOW-V | RCT | 1,334 | Completed 60-month FU available | TLF at 12 Months |
| BIO-RESORT | RCT | 3,514 | Completed 60-month FU available | TVF at 12 Months |
| BIONYX | RCT | 2,488 | 36-month FU available | TVF at 12 Months |
| BIOSCIENCE | RCT | 2,119 | Completed, 60-month FU available | TLF at 12 months |
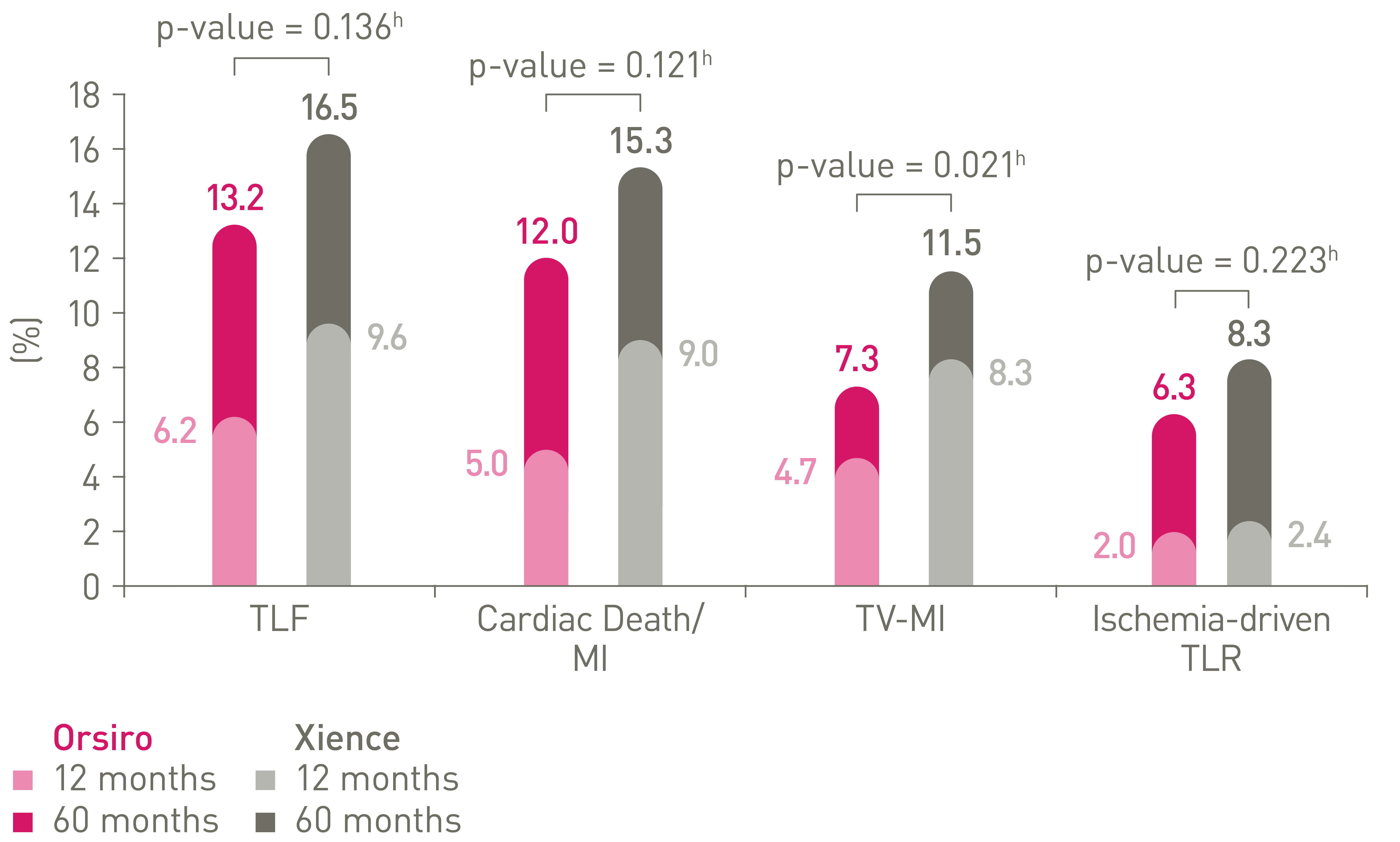
TLF and components out to 5 years16,17,18

ST events out to 5 years16