Outstanding patient outcomes
Outstanding patient outcomes
Orsiro has an extensive clinical program and shows outstanding clinical results with more than 55,000 patients enrolled1.

Outstanding patient outcomes
Orsiro has an extensive clinical program and shows outstanding clinical results with more than 55,000 patients enrolled1.
BIOFLOW-V (n = 1,334) FDA pivotal trial
David E. Kandzari, MD, Atlanta, USA
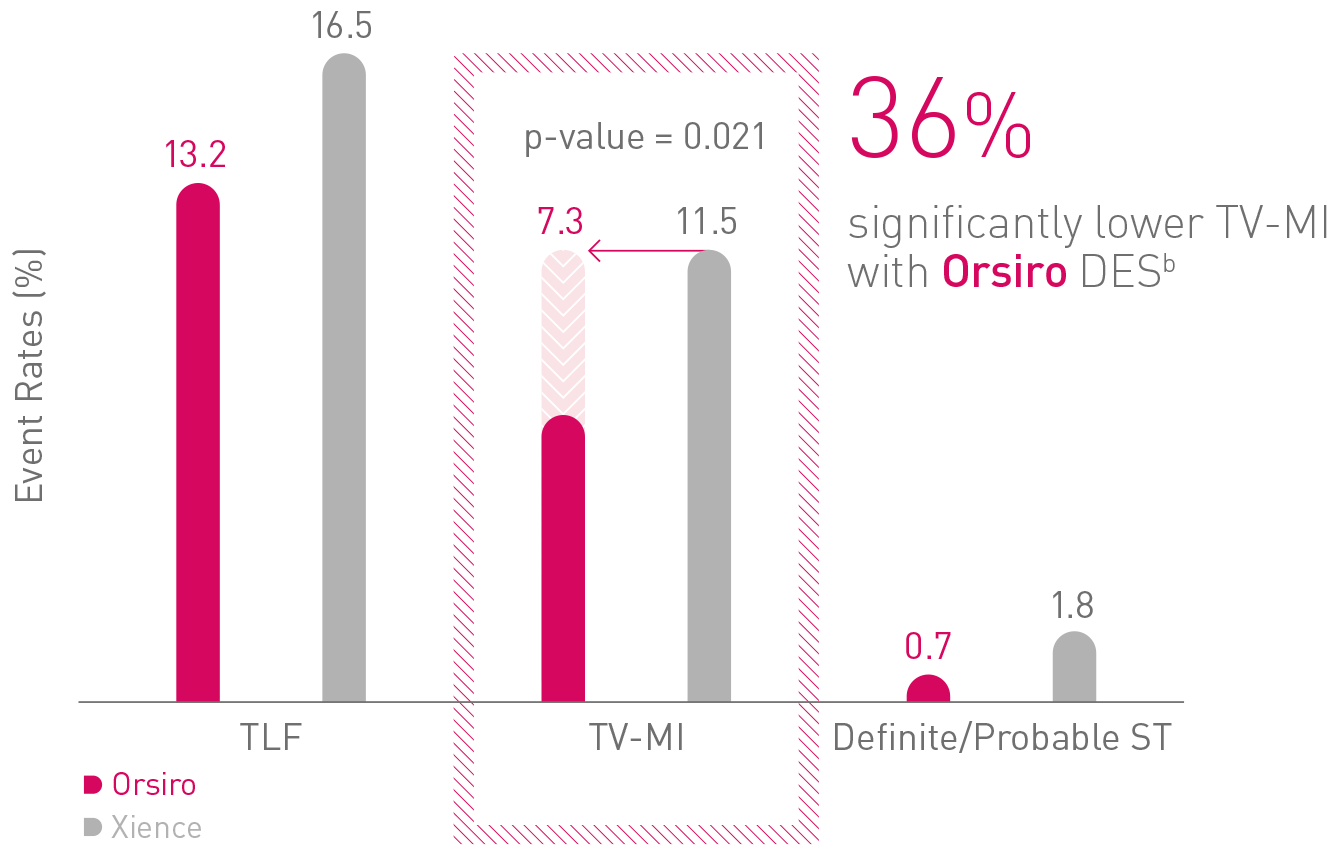
David E. Kandzari, MD, Atlanta, USA
TLF – Target Lesion Failure; TV-MI - Target Vessel Myocardial Infarction; ST – Stent Thrombosis2
a Based on statistically significant lower TV-MI and late/very late definite/probable ST rates through 5 years for Orsiro vs Xience in the BIOFLOW-V trial; b In comparison to Xience, based on BIOFLOW-V 5-year results.
1. BIOTRONIK data on file, status January 2020; 2. Kandzari D Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents versus Thin Durable Polymer Everolimus-Eluting Stents for Coronary Revascularization: Final 5-year Outcomes from the Randomized BIOFLOW V Trial. Presented at CRT 2022. Presented information is based on unrounded values.
Orsiro and Orsiro Mission are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
Xience is a trademark or registered trademark of the Abbott Group of Companies.
© 2022 BIOTRONIK AG – All rights reserved.
Specifications are subject to modification, revision and improvement.