

BIOFLOW-V (n = 1,334) FDA pivotal trial
BIOFLOW-V (n = 1,334) FDA pivotal trial
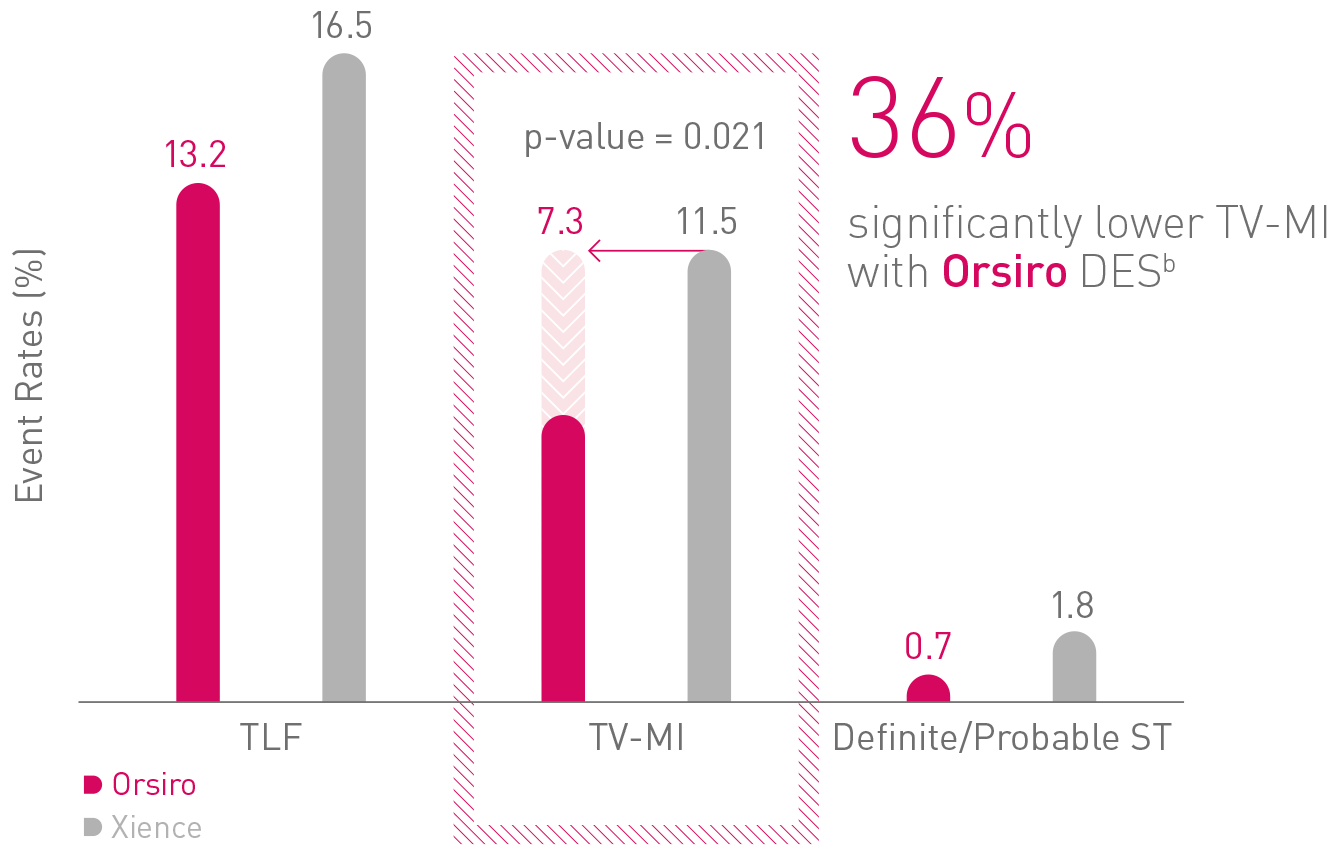
5-year results: Orsiro - pushing the boundaries of performance2,a
PRINCIPAL INVESTIGATOR OF THE FDA PIVOTAL BIOFLOW-V TRIAL PRESENTS 5-YEAR OUTCOMES2
David E. Kandzari, MD, Atlanta, USA
PRINCIPAL INVESTIGATOR OF THE FDA PIVOTAL BIOFLOW-V TRIAL PRESENTS 5-YEAR OUTCOMES2
David E. Kandzari, MD, Atlanta, USA
TLF – Target Lesion Failure; TV-MI - Target Vessel Myocardial Infarction; ST – Stent Thrombosis2
a Based on statistically significant lower TV-MI and late/very late definite/probable ST rates through 5 years for Orsiro vs Xience in the BIOFLOW-V trial; b In comparison to Xience, based on BIOFLOW-V 5-year results.
1. BIOTRONIK data on file, status January 2020; 2. Kandzari D Ultrathin Bioresorbable Polymer Sirolimus-Eluting Stents versus Thin Durable Polymer Everolimus-Eluting Stents for Coronary Revascularization: Final 5-year Outcomes from the Randomized BIOFLOW V Trial. Presented at CRT 2022. Presented information is based on unrounded values.
Orsiro and Orsiro Mission are trademarks or registered trademarks of the BIOTRONIK Group of Companies.
Xience is a trademark or registered trademark of the Abbott Group of Companies.
© 2022 BIOTRONIK AG – All rights reserved.
Specifications are subject to modification, revision and improvement.
