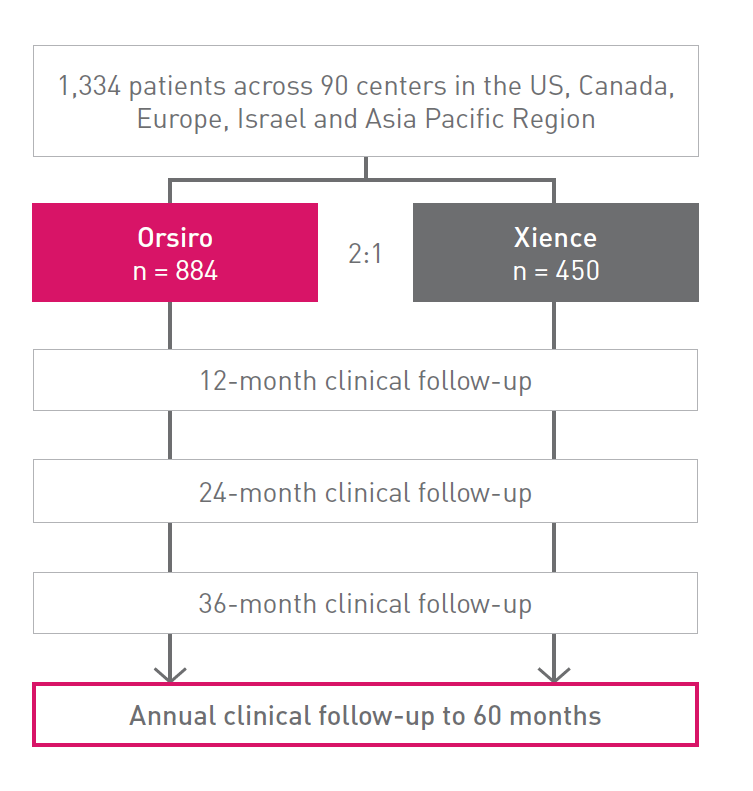
BIOFLOW-V
Comparison of Ultrathin strut Orsiro® Bioresorbable Polymer DES to Xience Durable Polymer DES at 60 Months1
PRINCIPAL INVESTIGATOR OF THE FDA PIVOTAL BIOFLOW-V TRIAL PRESENTS 5-YEAR OUTCOMES
David E. Kandzari, MD, Atlanta, USA
Conclusions
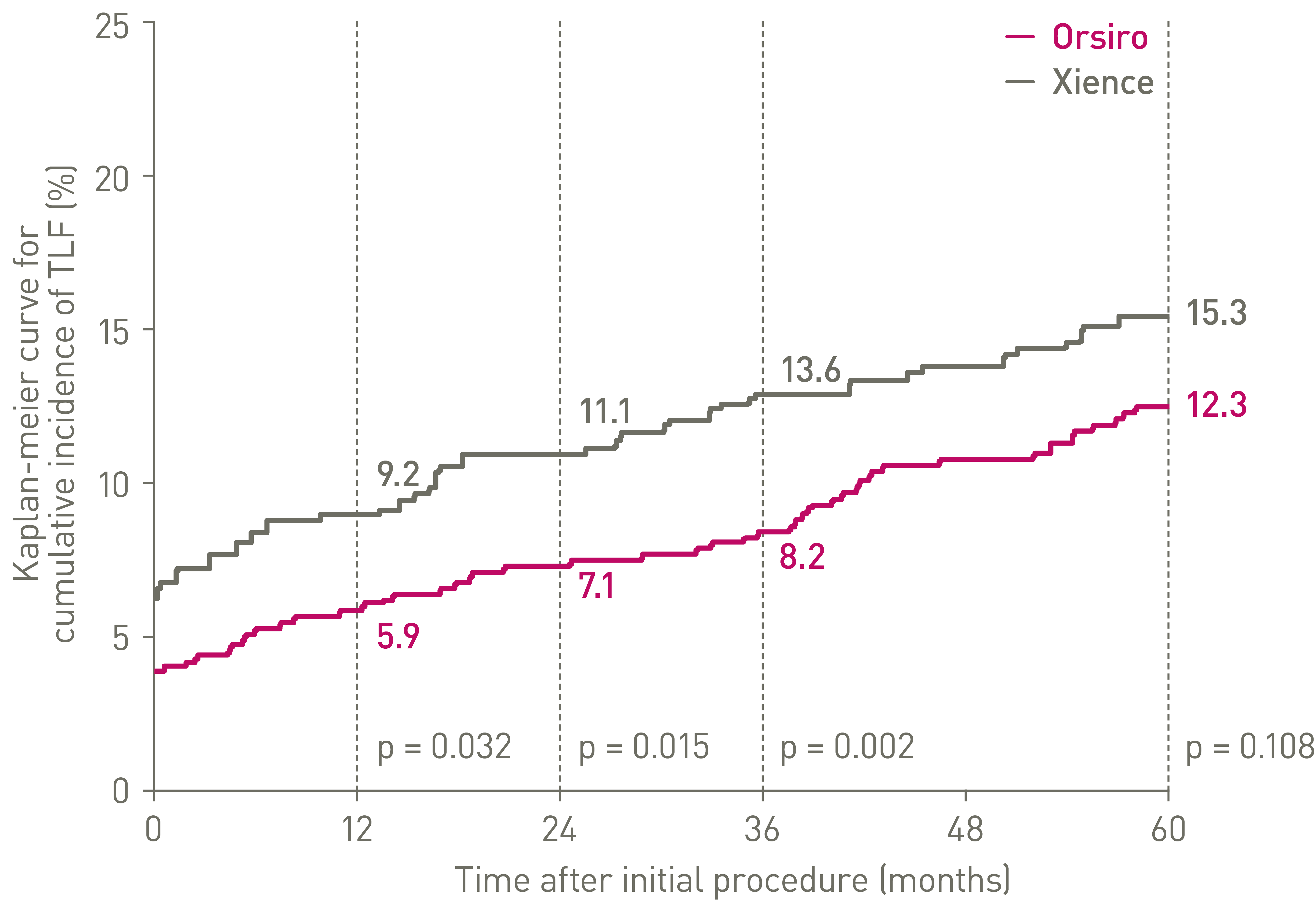
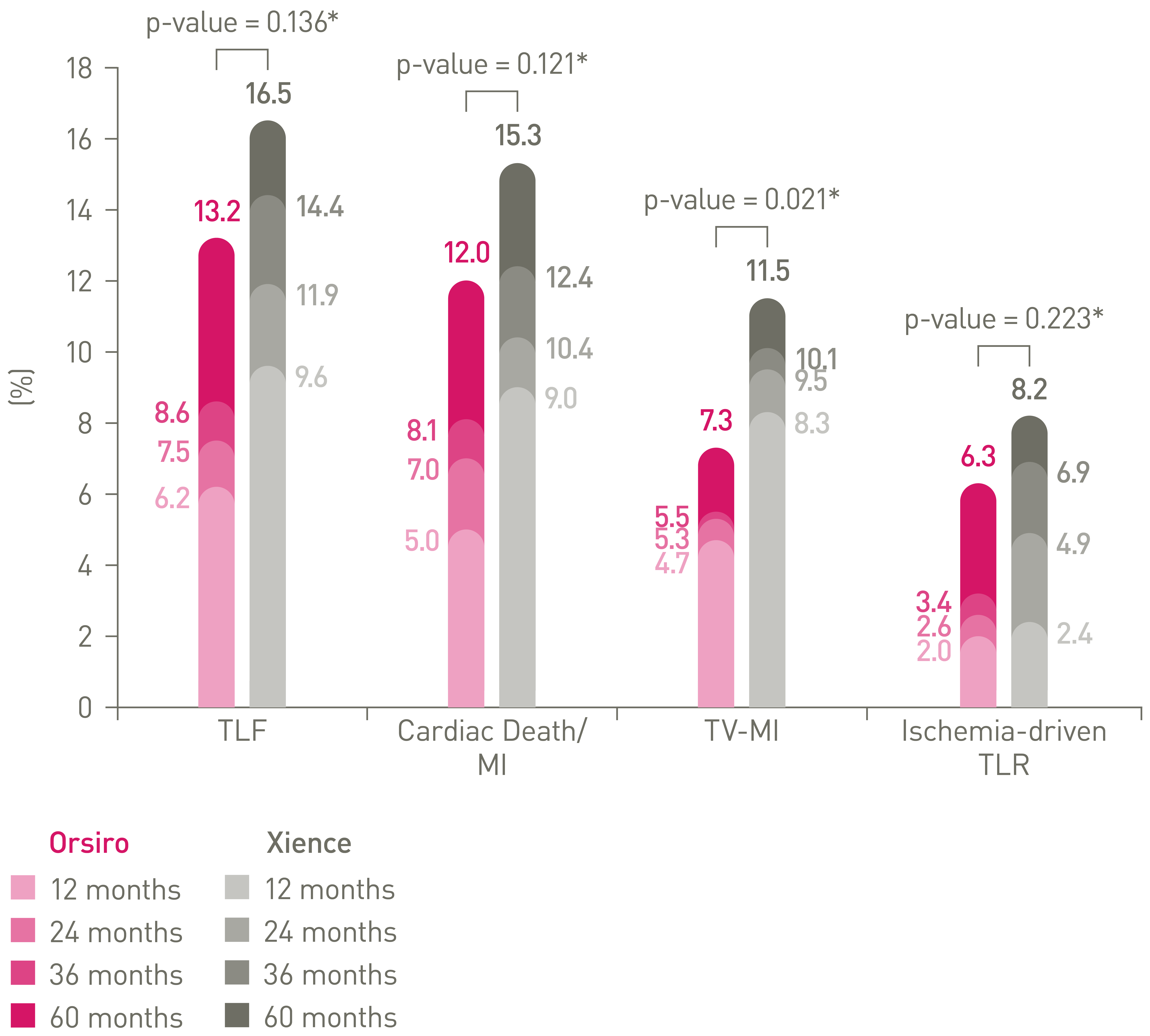
• Orsiro ultrathin struts DES outperformed Xience at 1-year and sustained performance up to 5 years:
• 20% lower Target Lesion Failure (p = 0.136)
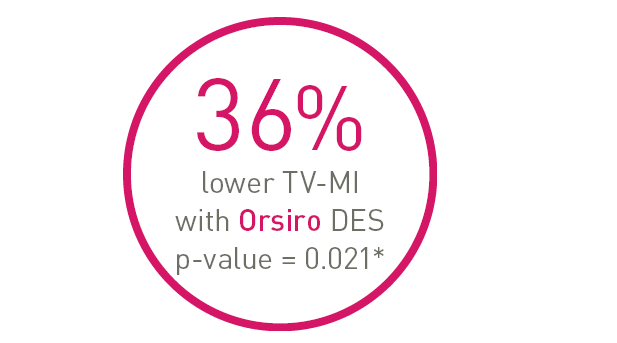
• 36% lower Target Vessel Myocardial Infarction (p = 0.021)
• 23% lower Ischemia-Driven TLR (p = 0.223)
• 22% lower Cardiac Death/Myocardial Infarction (p = 0.121)
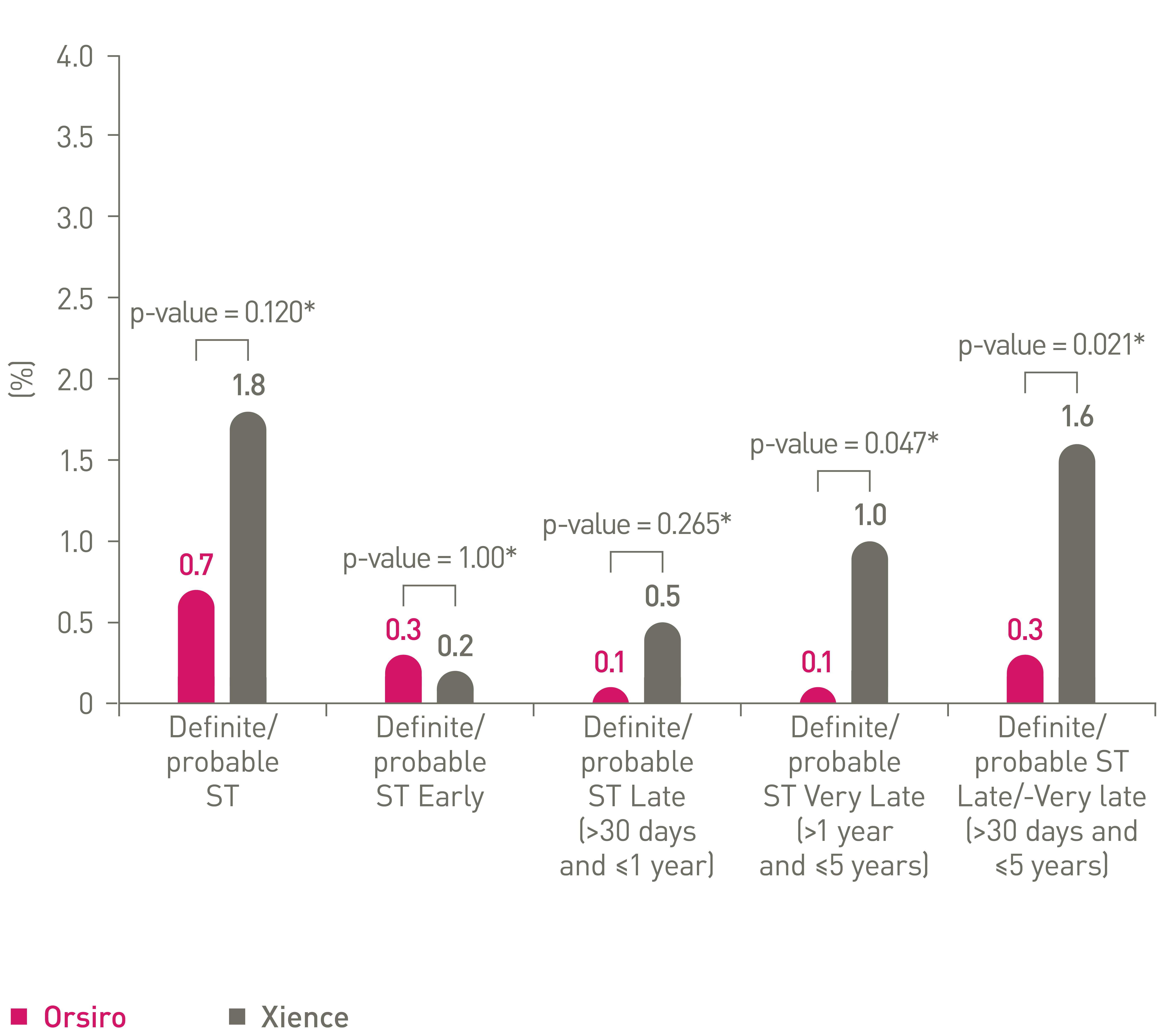
• Orsiro showed a 0.7% definite/probable stent thrombosis rate overall through 5 years: 64% lower compared to Xience (p=0.120)
•83% lower late/very late definite/prob ST (p = 0.021)