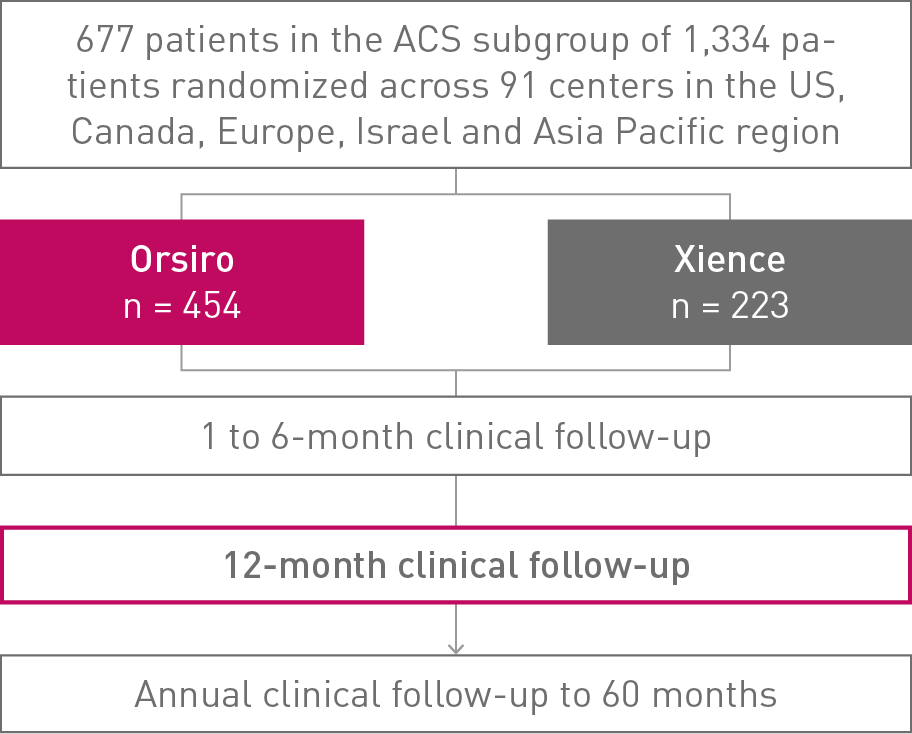
BIOFLOW-V
Comparison of Ultrathin Orsiro® Bioresorbable Polymer DES to Xience* Durable Polymer DES Acute Coronary Syndrome (ACS) Subgroup Analysis at 12 months
BIOFLOW-V: FDA PIVOTAL TRIAL AT 36 MONTHS
Conclusions
• More than 50% of the patients treated in the BIOFLOW-V study presented with ACSΔ
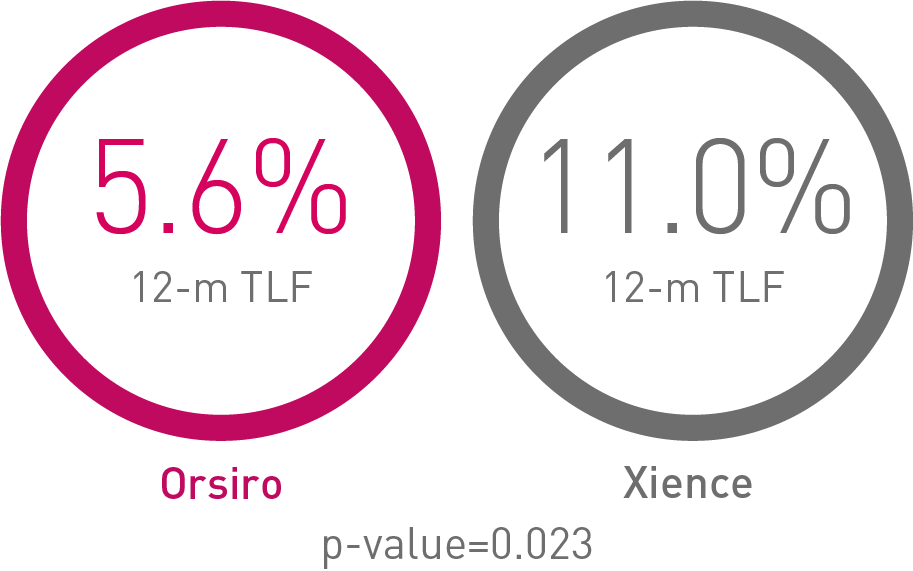
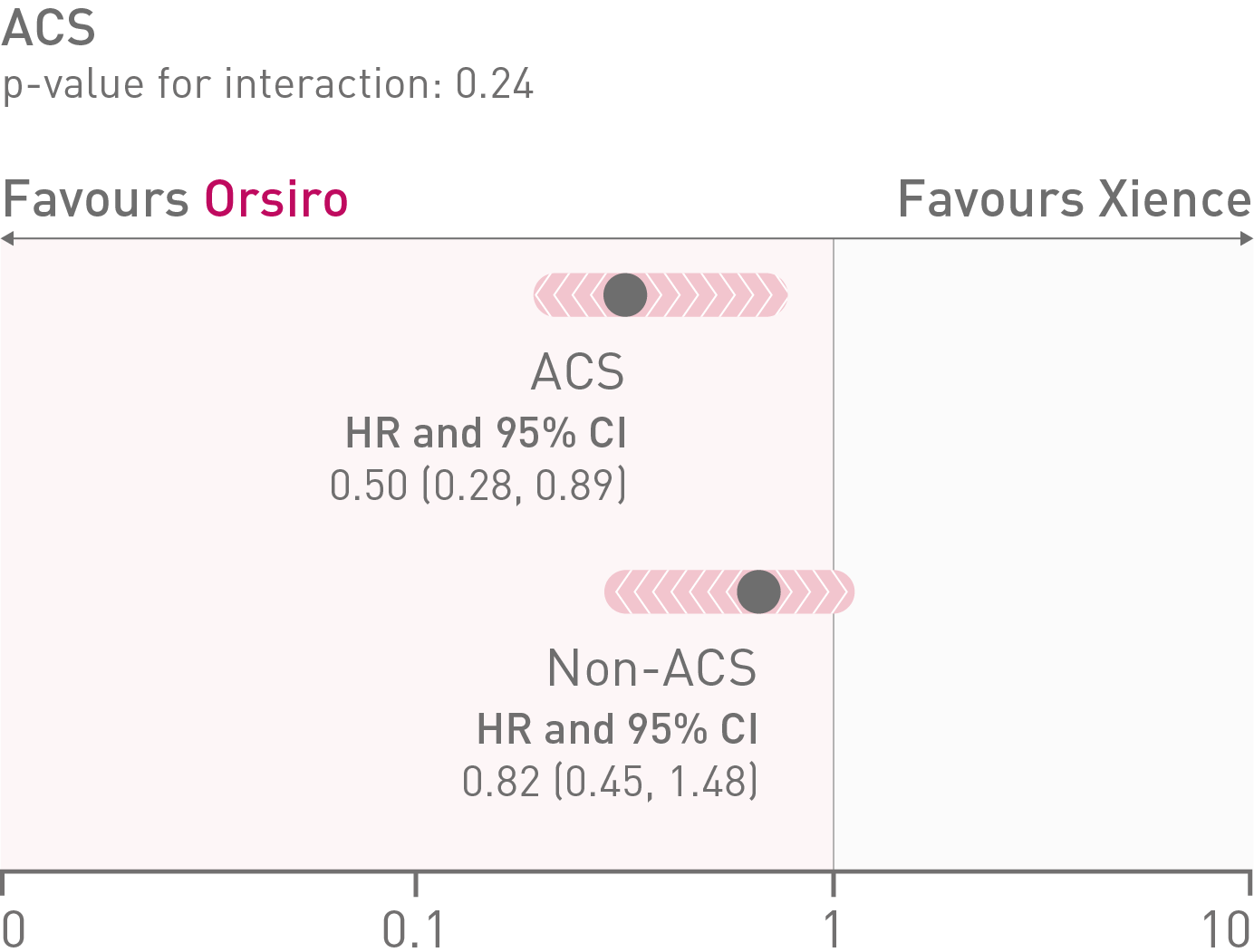
• In the ACS subgroup analysis, Orsiro® demonstrated statistically significantly lower Target Lesion Failure (TLF) rates compared to Xience at 12 months (Orsiro 5.6%, Xience 11.0%, p-value = 0.023)
• Procedure success was significantly higher with Orsiro (Orsiro 94.7%, Xience 89.7%, p-value = 0.023), mainly driven by significantly lower peri-procedural Myocardial Infarction (MI)