BIO-RESORT
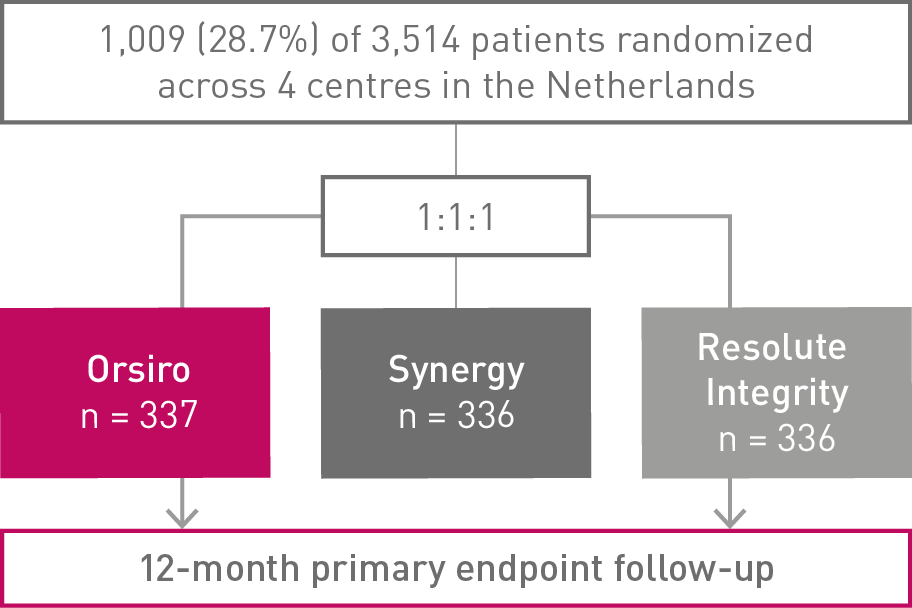
12-month High-Bleeding Risk (HBR) subgroup analysis of the BIO-RESORT trial, RCT Orsiro and Synergy vs. Resolute Integrity
Orsiro® DES - Extensive Clinical Program
Conclusions
• Almost 29% of the BIO-RESORT all-comers had a High-Bleeding Risk (HBR)
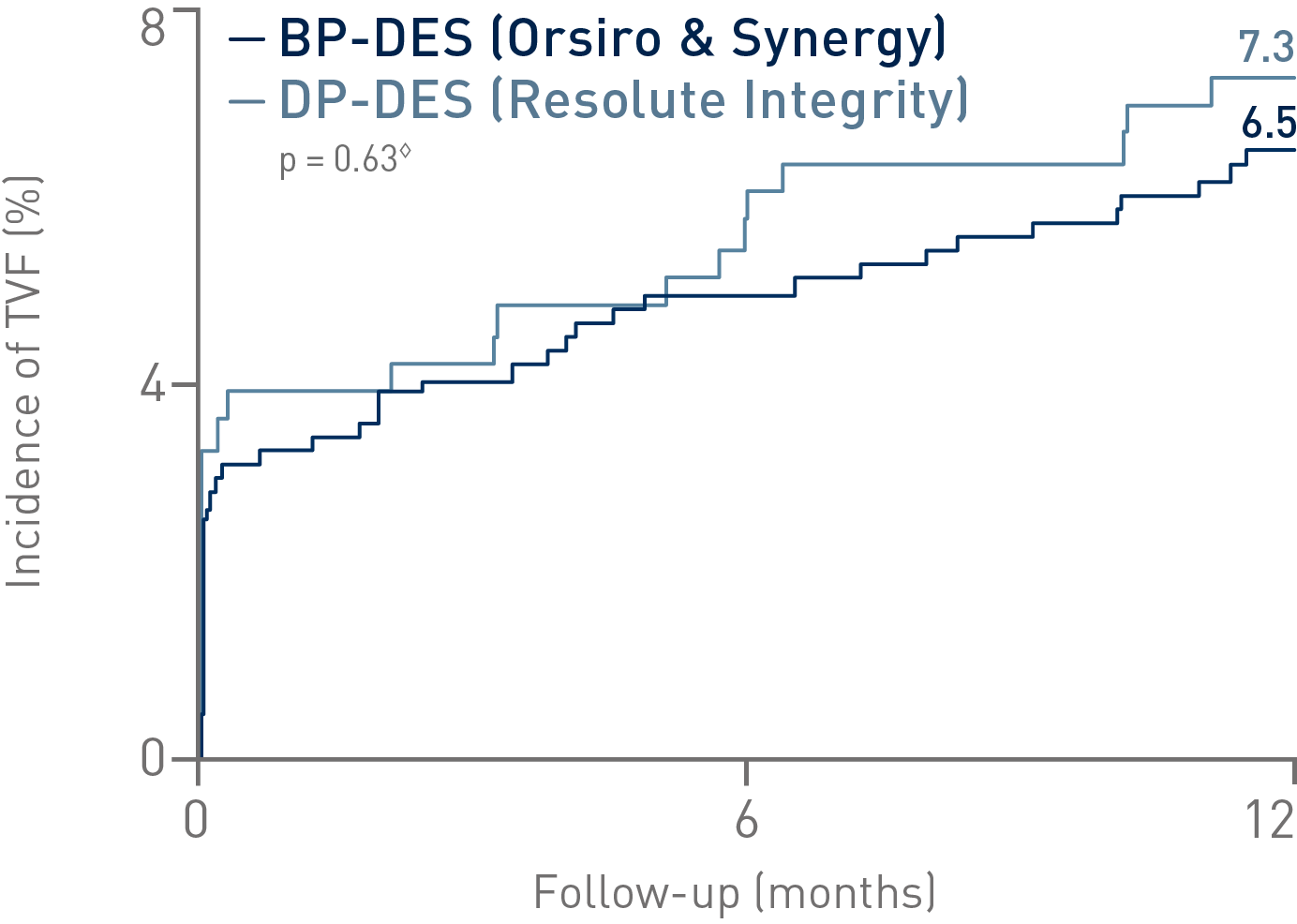
• In this subanalysis (n = 1,009), the Bioabsorbable Polymer DES (BP-DES) arm, including Orsiro®, showed numerically lower event rates of the primary composite endpoint Target Vessel Failure (TVF) compared to Durable Polymer DES (DP-DES)
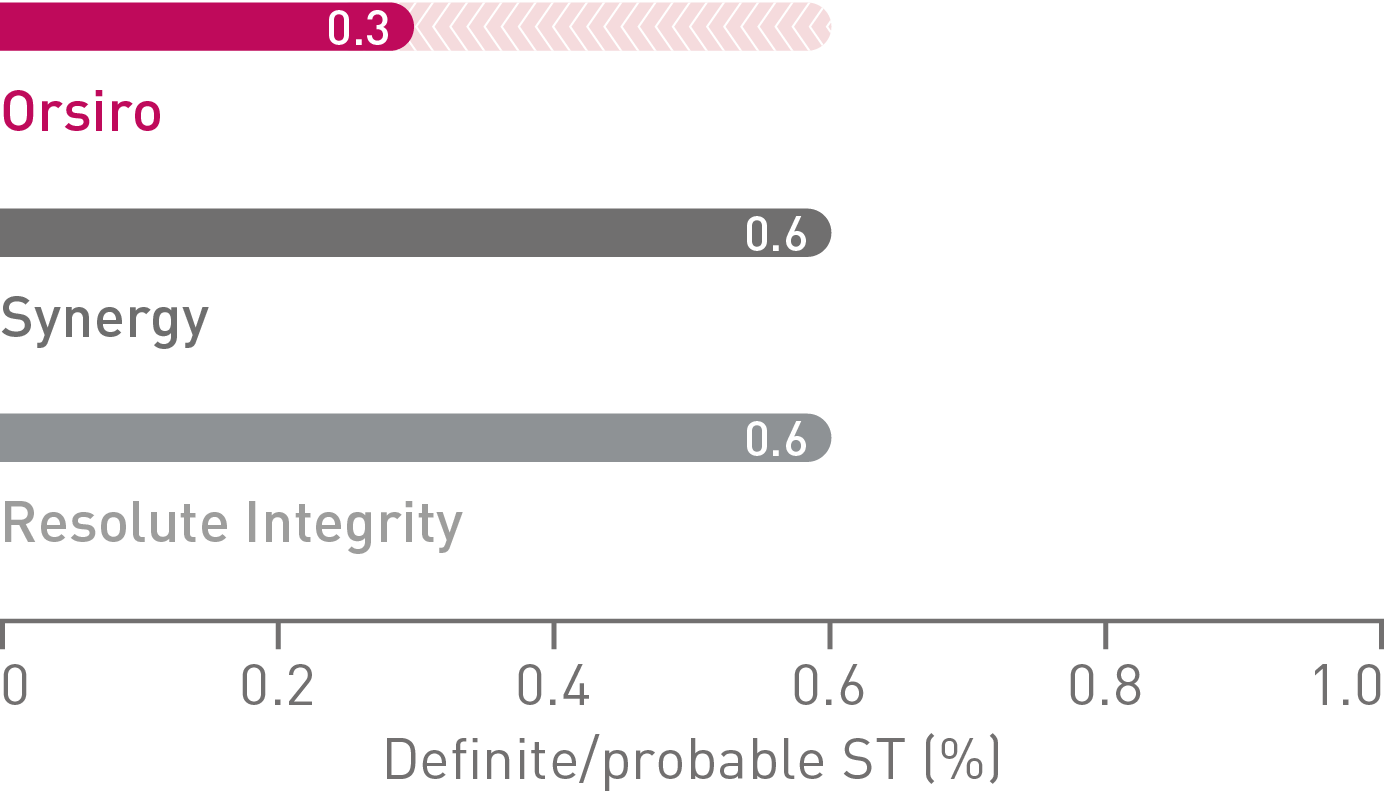
• On a product level, Orsiro alone demonstrated a numerically lower TVF rate (6.0%) than Synergy* (6.9%), and Resolute Integrity** (7.3%) in HBR patients, respectively. The differences did not reach statistical significance