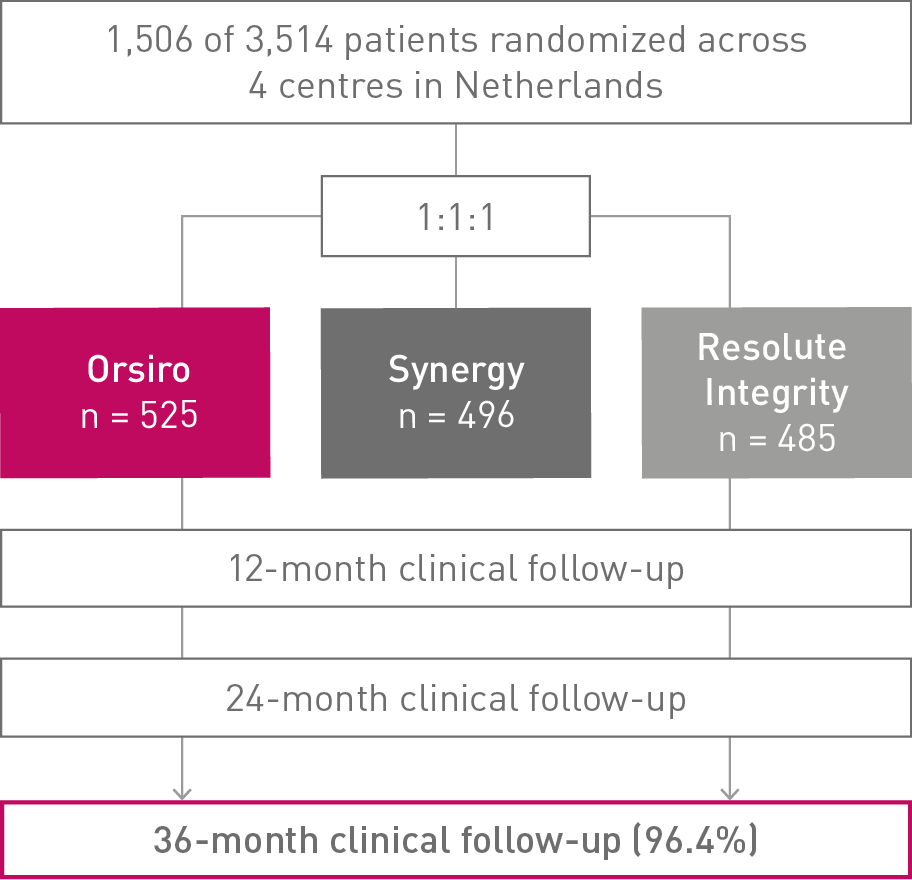
BIO-RESORT
36-month clinical follow-up results of the Small Vessel subgroup
Orsiro® DES - Extensive Clinical Program
Conclusions
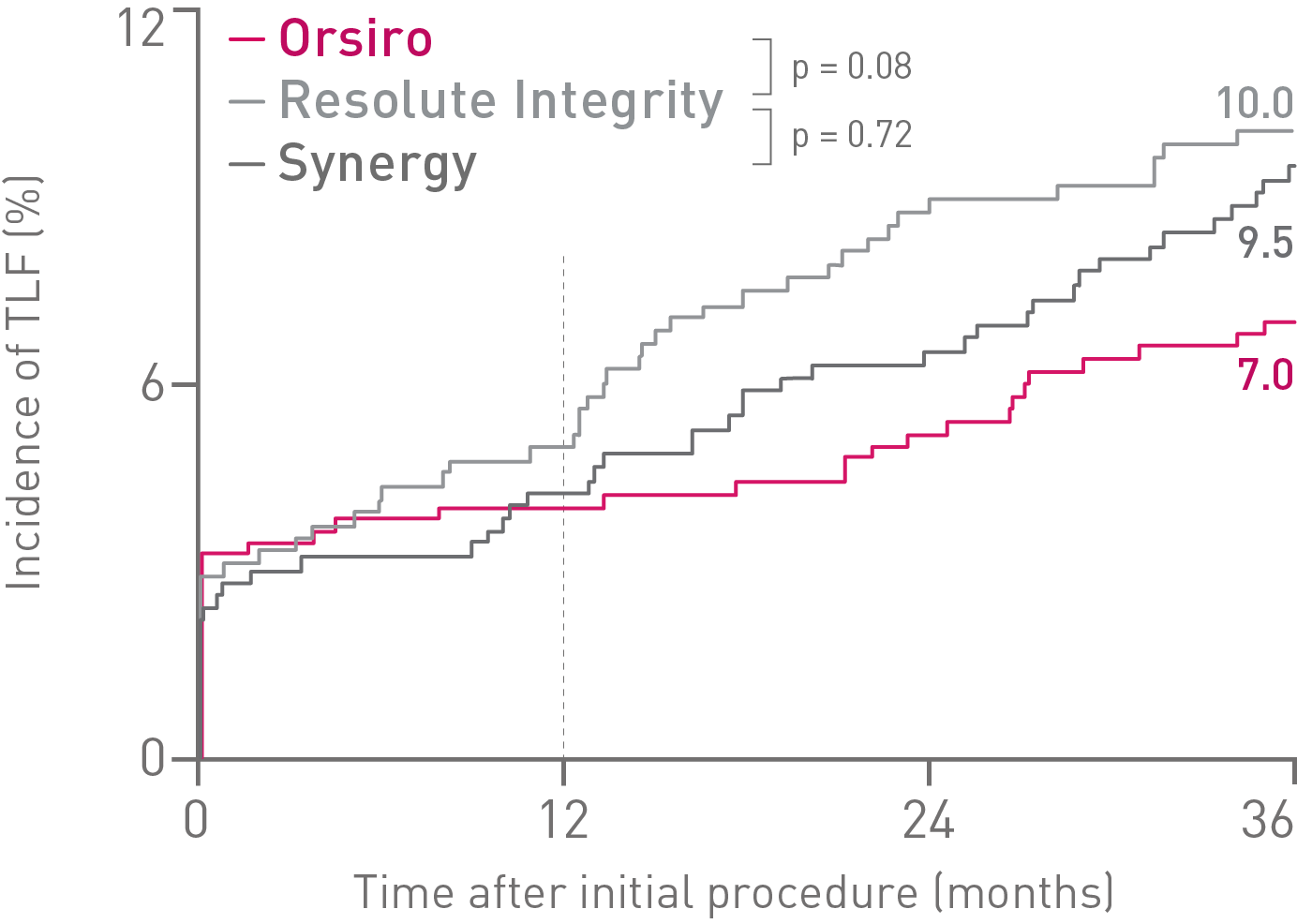
• At 36 months in the small vessel subgroup, Orsiro® shows a trend towards lower rates of Target Lesion Failure (TLF) and Stent Thrombosis (ST) compared to both Resolute Integrity* and Synergy**.
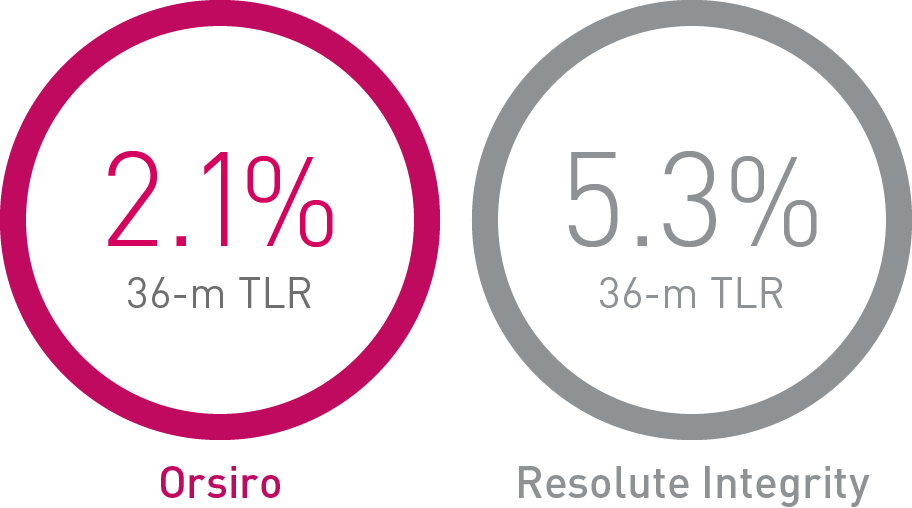
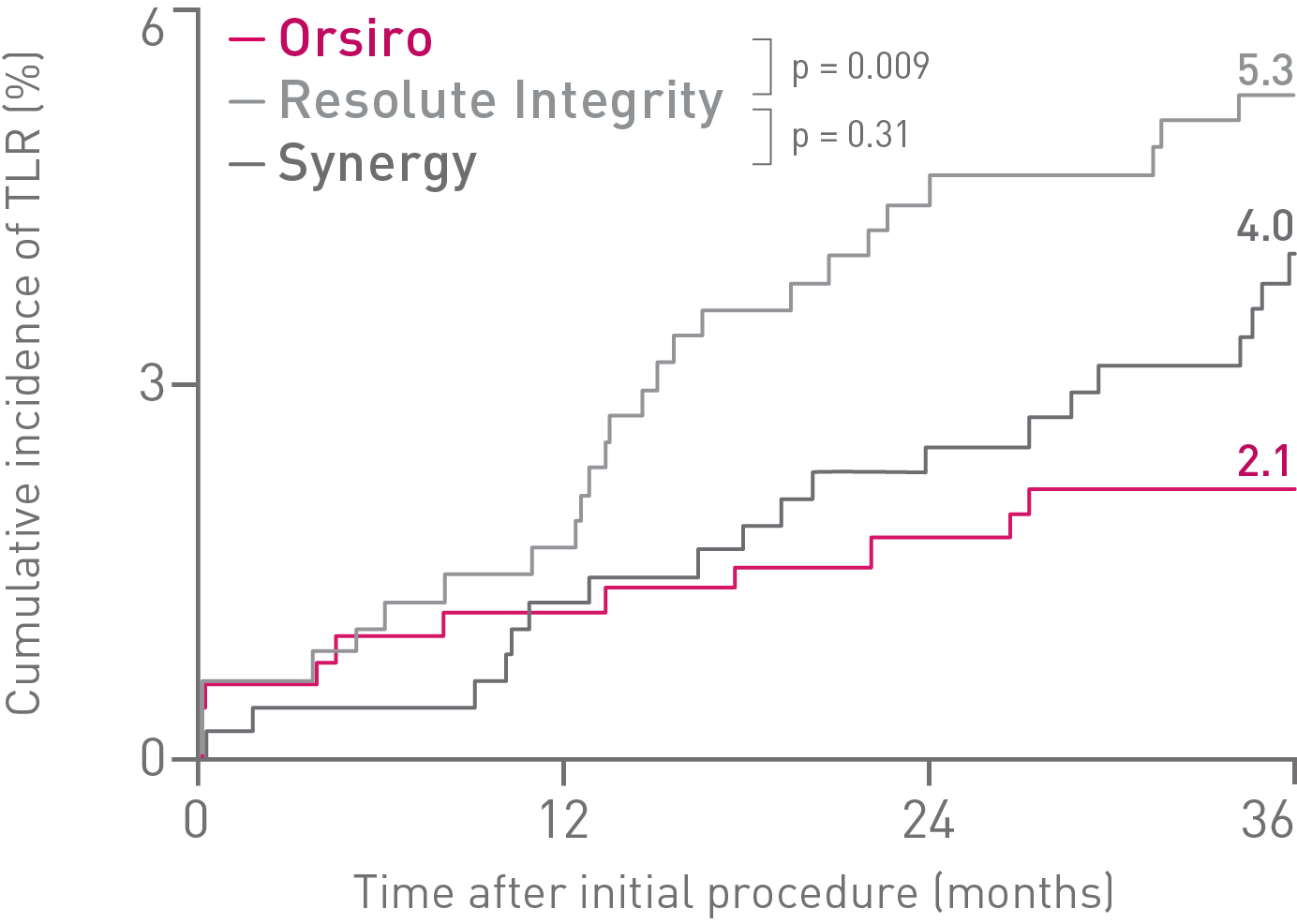
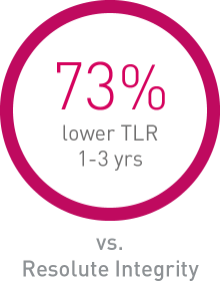
• Orsiro demonstrates significantly lower rates in Target Lesion Revascularization (TLR) compared to Resolute Integrity and numerically lower rates compared to Synergy.
• Orsiro’s ultrathin struts may contribute to a lower repeat revascularization risk in patients with small target vessels.